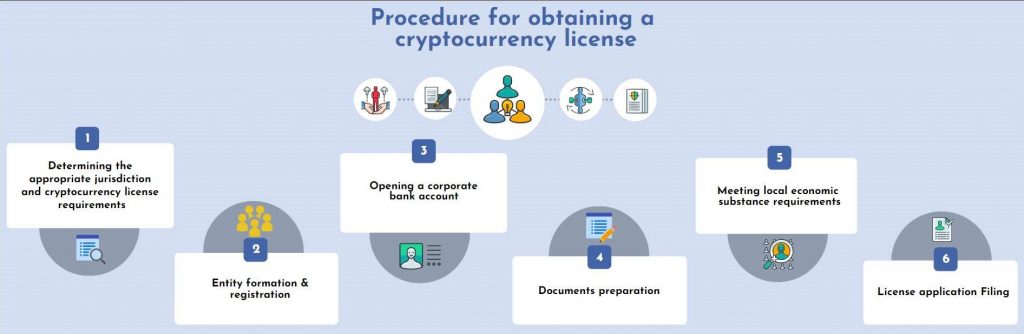
Where to obtain a cryptocurrency license
Keep in mind the obligatory licensing criteria pertaining to presence in the nation when picking a country to license bitcoin operations (Japanese rules require an office in the country and recruitment of employees, so it is not possible to obtain a license in that country remotely). Service fees and necessary contributions to the permitted capital (the range of prices for the licensing process depends on the volume of government fees and associated costs, the total amount can be several tens of thousands of dollars, and the authorized capital – up to hundreds of thousands). The length of the process (up to several months). The nations that have little impact on the global financial system and actively aim to draw investment to their borders are the most convenient for acquiring a license for crypto-exchange activities today.